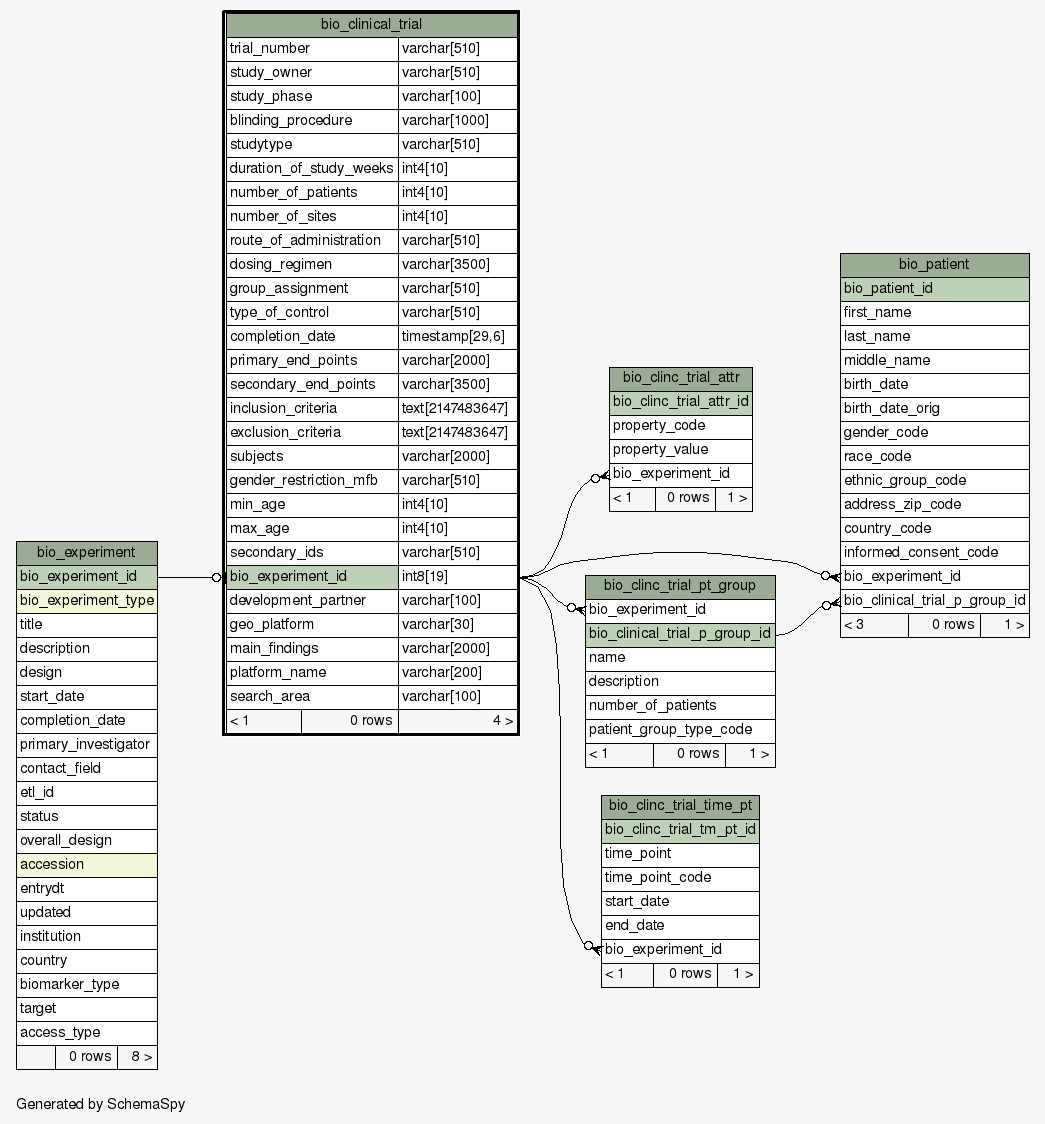
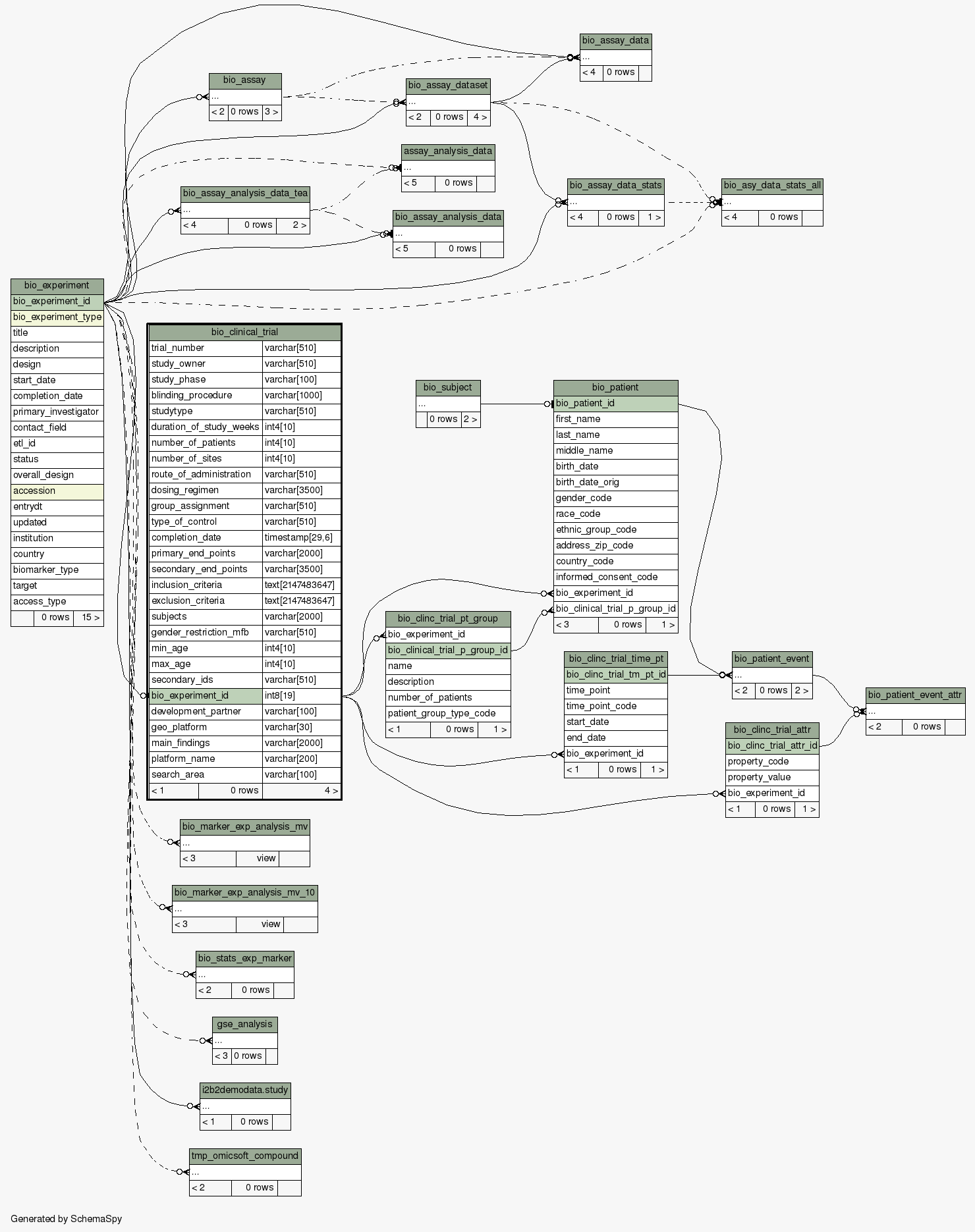
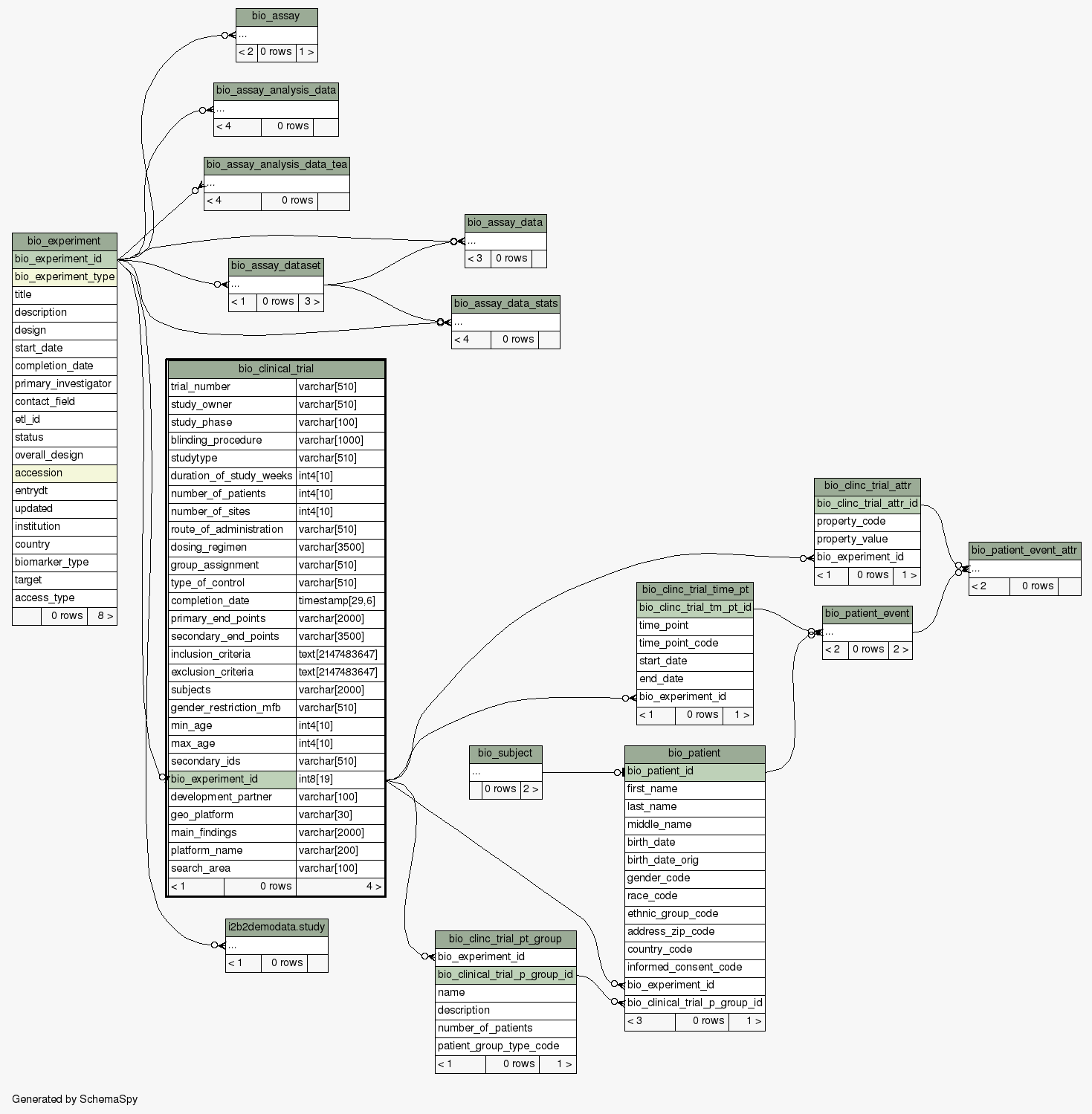
| Column |
Type |
Size |
Nulls |
Auto |
Default |
Children |
Parents |
| trial_number |
varchar |
510 |
√ |
|
null |
|
|
| study_owner |
varchar |
510 |
√ |
|
null |
|
|
| study_phase |
varchar |
100 |
√ |
|
null |
|
|
| blinding_procedure |
varchar |
1000 |
√ |
|
null |
|
|
| studytype |
varchar |
510 |
√ |
|
null |
|
|
| duration_of_study_weeks |
int4 |
10 |
√ |
|
null |
|
|
| number_of_patients |
int4 |
10 |
√ |
|
null |
|
|
| number_of_sites |
int4 |
10 |
√ |
|
null |
|
|
| route_of_administration |
varchar |
510 |
√ |
|
null |
|
|
| dosing_regimen |
varchar |
3500 |
√ |
|
null |
|
|
| group_assignment |
varchar |
510 |
√ |
|
null |
|
|
| type_of_control |
varchar |
510 |
√ |
|
null |
|
|
| completion_date |
timestamp |
29,6 |
√ |
|
null |
|
|
| primary_end_points |
varchar |
2000 |
√ |
|
null |
|
|
| secondary_end_points |
varchar |
3500 |
√ |
|
null |
|
|
| inclusion_criteria |
text |
2147483647 |
√ |
|
null |
|
|
| exclusion_criteria |
text |
2147483647 |
√ |
|
null |
|
|
| subjects |
varchar |
2000 |
√ |
|
null |
|
|
| gender_restriction_mfb |
varchar |
510 |
√ |
|
null |
|
|
| min_age |
int4 |
10 |
√ |
|
null |
|
|
| max_age |
int4 |
10 |
√ |
|
null |
|
|
| secondary_ids |
varchar |
510 |
√ |
|
null |
|
|
| bio_experiment_id |
int8 |
19 |
|
|
|
|
|
| development_partner |
varchar |
100 |
√ |
|
null |
|
|
| geo_platform |
varchar |
30 |
√ |
|
null |
|
|
| main_findings |
varchar |
2000 |
√ |
|
null |
|
|
| platform_name |
varchar |
200 |
√ |
|
null |
|
|
| search_area |
varchar |
100 |
√ |
|
null |
|
|
Table contained 0 rows at Wed Jun 26 00:10 CEST 2019
|